EFFECTIVE ONLY JUNE 3 – AUGUST 30, 2020
Please note, information on this page was only relevant during June 3 – August 30, 2020. Please visit the Research Operations webpage for current details about the return of onsite research to campus.
- Survey Results: Restart Progress
-
Research-Related Staff and Postdocs Comfortable with Restart Efforts and Communications
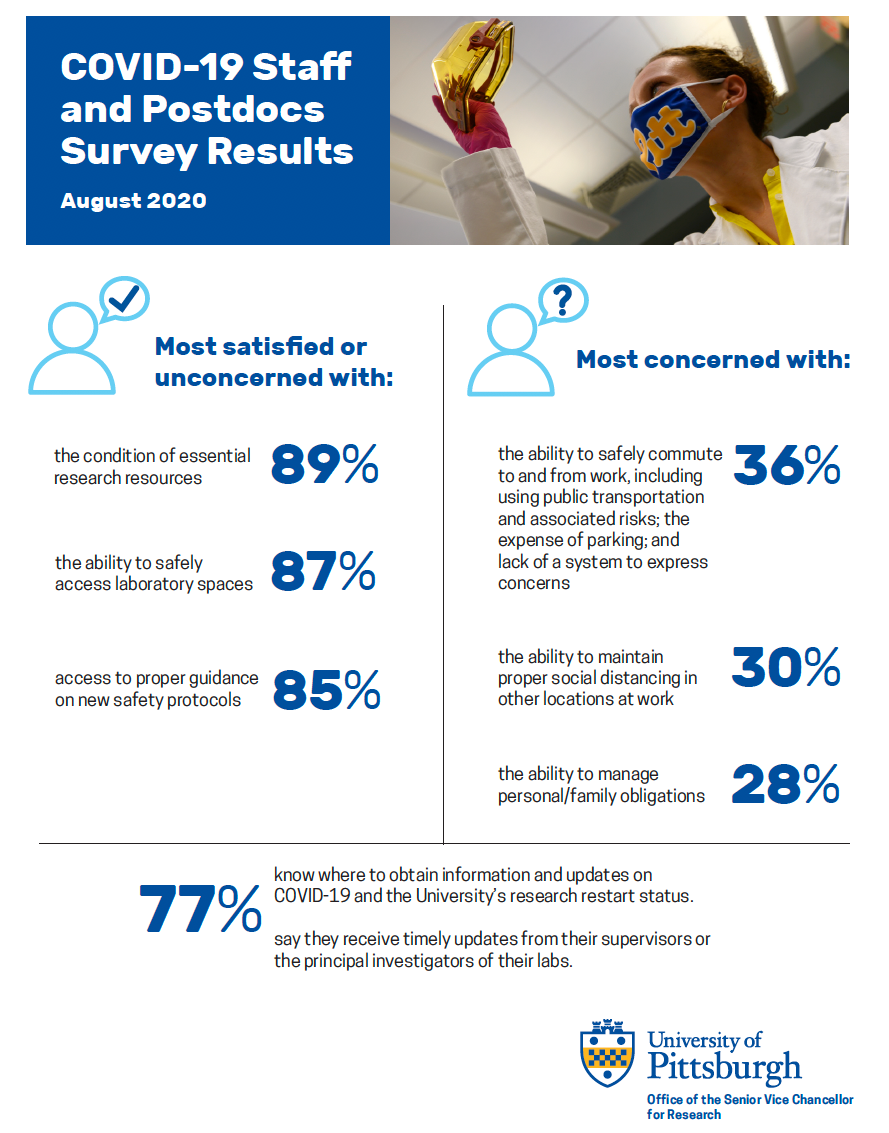
To monitor progress on Pitt’s research restart initiative from the perspective of research-related staff and postdocs, three senior leaders at Pitt asked them to weigh in on how policy and practice changed since they were last surveyed in May.
Senior Vice Chancellor for Research Rob Rutenbar, Acting Senior Vice Chancellor for Business and Operations and Vice Chancellor of Human Resources David N. DeJong, and Vice Provost for Graduate Studies Amanda Godley sent the survey on August 11 to 1,500 staff and postdocs involved in research-related work. About 25% responded.
Two-thirds of the respondents said that the restart had gone at least as well as they expected, or better. Half of the respondents expressed concern about their general safety, including wearing a mask properly, social distancing, using public transportation, and the lack of an enforcement mechanism to keep people accountable.
In more detail, research-related staff and postdocs offered the following (click on the graphic below to open a full-size PDF):
The three top sources of information on the University’s COVID-19 updates have been the COVID website, Pitt’s general website, and researchers’ department deans or chairs.
The survey results validate that employees are using Pitt’s communications channels, and that efforts should continue to improve how information is made available to the University community. Pitt’s main coronavirus webpage connects members of the University community to guidance on a broad range of topics, including specifics about returning to campus and tips on traveling modes and their risks.
Since the survey was introduced, the University has addressed many of the safety, childcare and transportation concerns expressed with the following initiatives or programs:
- Developed and implemented training for employees and supervisors on COVID-19 health and safety measures (August 10)
- Implemented Pandemic Safety Network consisting of both Building Safety Concierge Program and Pandemic Safety Ambassador Network (August)
- Offered childcare resources
- UCDC has reopened with limitations (August 11)
- Held two webinars on childcare opportunities throughout southwestern Pennsylvania (offered only to Pitt community July 31 and September 1)
- Offered more transportation resources
- Implemented temporary suspension of parking permits for those working remotely and offered resources for daily parking options (since April)
- Implemented program for free rides with Healthy Ride bike network (August 5)
- Research Standards and Guidelines
-
New operational guidance for research is now available to offer Pitt researchers a proactive, flexible and resilient framework to enable research work as we all accommodate the circumstances of the pandemic.
Four Standards and Guidelines have been written to apply to each of the University’s COVID-19 Operational Postures (High Risk, Elevated Risk, and Guarded Risk) to help researchers mitigate impacts throughout the duration of the pandemic. They are found on the COVID-19 Standards and Guidelines page.
The Standards and Guidelines offer guidance on:
- Research Operations and Governance: These guidelines define Conduct of Research templates for use at the school level and Conduct of Research plans for individual principal investigators. The guidelines establish the requirement that every investigator’s Conduct of Research plan explains how their research activities will be conducted safely under all three of the University’s COVID-19 operational postures and how transitions between postures will be managed.
- Research Operations Prioritization: These guidelines establish the principle that operational activities will vary by broad categories of research. For example, during the pandemic, clinical research will have different needs than will libraries or studio spaces. The guidelines define priority research and research support activities that will be allowed to continue under all three of the University’s COVID-19 operational postures.
- Fieldwork and Research Travel: These guidelines establish the principle that local conditions will govern the Conduct of Research plans for investigators engaged in fieldwork and that the health and safety guidelines implemented in the field must meet or exceed those of the University of Pittsburgh. These guidelines further define some of the responsibilities of investigators in the field with respect to the health and safety of their research team.
- Human and Animal Research Subjects: The guidelines codify the Institutional Review Board’s tiers for protocols based on direct benefit to research participants. They further identify situations when new petition or survey submission to the Board are required. Links are given to the DLAR documents governing operations of their animal facilities during the high-risk posture as well as the elevated and guarded risk postures.
These documents codify the work of the Research Restart Taskforce and its 7 working groups, which involved more than 100 faculty and staff. The final Research Standards and Guidelines can be found on the Office of Policy Development and Management’s COVID-19 Standards and Guidelines page, along with relevant health, employee, facility, meetings, events and travel standards and guidelines.
- Governance and Process
-
Deans, Institute Directors, and Regional Campus Presidents play a central role in translating campus-wide guidance into direction for their schools and for having final authority to approve the restart activities of their faculty. A more detailed explanation of the process follows:
- Step 1: Deans, Institute Directors and Regional Campus Presidents are responsible for restarting research in their units based on standards, guidelines and planning templates first developed at campus-level by the Emergency Operations Center (EOC) and the Research Restart Task Force Working Groups. First versions of these standards and guidelines, organized by academic discipline and research area, are available below. Schools, Institutes and Regional Campuses may need to use several of these guidelines. They may also need to adjust or modify these guidelines to meet local, research specific needs; if so, Deans, Directors and Regional Campus Presidents should submit their proposed revisions to the OSVCR Restart Standards & Clearance Committee, via svcrclearance@pitt.edu, for review.
- Step 2: The Research Restart Standards & Clearance Committee will ensure that each Dean’s or President’s context-specific research restart plan(s) comply with campus-wide standards and guidelines.*
- Step 3: Once approved, Dean, Institute Directors or Regional Campus Presidents will provide their suite of restart plans to chairs and faculty/principal investigators (PIs).
- Step 4: Faculty/Principal Investigators will create their individual restart plans and show how they have applied the appropriate context-appropriate research restart plan to their specific work process and research spaces. Once the individual restart plans are completed, a Research Restart Plan Review Checklist will be completed and attached.
- Step 5: Faculty/PI individual restart plans and Research Restart Plan Review Checklist will be reviewed and approved the Dean, Institute Directors, Regional Campus Presidents or their designees (e.g., Associate Deans for Research or Department Chairs). Follow your school/unit process for submission of these two documents for approval.
For shared resources, research cores, and multi-PI lab spaces, the process, although similar to individual PI research programs, requires coordination, input, and a clear path of responsibility for the research plans as indicated in the “Detailed Review by 'Scale' of Research Restart”.
*The Standards & Clearance Committee will also serve as a forum for PIs to appeal the decisions of their Dean, Regional Campus President, or Institute Director and will only review and approve the restart plans of resources or individual labs in cases of special complexity, such as plans for labs that span school boundaries.
- School and Unit Restart Plans
-
Deans, Institute Directors, and Regional Campus Presidents are responsible for developing and obtaining approval of a restarting research plan(s) for their unit(s). Contact your Dean or Associate Dean for Research, Institute Directors, and/or Regional Campus Presidents.
- Arts and Sciences
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- STEM Field Research Mitigation Plan
- STEM Individual Lab Research Restart Plan
- STEM Principles and Guidance
- STEM Shared Lab Space Mitigation Plan
- Business
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- Computing and Information
- Education
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- Engineering
- Health Sciences: Dental Medicine, Health and Rehabilitation Sciences, Pharmacy, Nursing, Public Health
- Law
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- Learning Research & Development Center (LRDC)
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- STEM Field Research Mitigation Plan
- STEM Individual Lab Research Restart Plan
- STEM Principles and Guidance
- STEM Shared Lab Space Mitigation Plan
- Libraries
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- Medicine
- Public and International Affairs
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- Social Work
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- University Center for Social and Urban Research (UCSUR)
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- University of Pittsburgh Bradford
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- STEM Field Research Mitigation Plan
- STEM Individual Lab Research Restart Plan
- STEM Principles and Guidance
- STEM Shared Lab Space Mitigation Plan
- University of Pittsburgh Greensburg
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- STEM Field Research Mitigation Plan
- STEM Individual Lab Research Restart Plan
- STEM Principles and Guidance
- STEM Shared Lab Space Mitigation Plan
- University of Pittsburgh Johnstown
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research Fieldwork
- Art, Humanities, Social Science, Business, Law (AHSSBL) Return to Research On Campus
- Art, Humanities, Social Science, Business, Law (AHSSBL) Studio Space Restart Plan
- Art, Humanities, Social Science, Business, Law (AHSSBL) Lab Space Restart Plan
- STEM Field Research Mitigation Plan
- STEM Individual Lab Research Restart Plan
- STEM Principles and Guidance
- STEM Shared Lab Space Mitigation Plan
- Arts and Sciences
- PI Checklist
-
Complete the information requested in the appropriate restart plan selected by your Dean, Institute Director, Regional Campus President. They will be informing you of the local review process in your school, institute or campus.
If your school, institute or regional campus plan does not have its own checklist, you may use the Research Restart Plan Review Checklist.
- Building Information
-
Before onsite research can begin, the building where the research is being conducted must have undergone preparation and readiness review by Pitt Facilities Management.
Note: Not all buildings are certified as ready for return, and be aware that “certified ready” does not mean “open”.
Facilities Management is working as expeditiously as possible to get buildings cleared for restart, so check back frequently as more buildings are continually becoming research ready.
- Human Resources Information
-
Check with your Dean or Associate Dean for Research, Institute Directors, and/or Regional Campus Presidents for HR guidance, or check the Human Resources COVID-19 page.
- Environmental Health and Safety Information
-
EH&S has assembled online educational resources for COVID-19 general information as well as specific guidance for putting on, wearing, and taking off face coverings (what is COVID-19, why do I need a face covering, and how can I protect myself and others?).
Please review all health and safety related information for research restart such as cleaning information, non-laboratory workplaces, laboratory information, and mitigation templates.
- PPE Guidance
-
The Research Restart Task Force surfaced the need for additional guidance for specific Personal Protective Equipment (PPE) use cases. Pitt Environmental Health and Safety (EH&S) has issued Full Guidance and Use Case Scenarios for Face Coverings and PPE.
EH&S Guidance for Face Coverings and Personal Protective Equipment clarifies the use of face coverings during the COVID-19 pandemic. The guidance also provides advice on use of face coverings vs PPE in cases where EH&S safety surveys and/or risk assessments have recommended use of task-specific PPE.
Information about orders for PPE, COVID-mitigation supplies, and cleaning supplies to be distributed from the centralized inventory by the Dietrich School Stockroom, is available on the PantherExpress website.
For instructions on how to obtain PPE and COVID-mitigation supplies for your research program, please contact your Departmental Administrator.
Several suggested online resources describing appropriate use of face coverings and gloves as well as demonstrations for properly putting on and taking off these items have been reviewed by Pitt EH&S, and links are also available on the Pitt EH&S website.
- Signage Templates
-
Facilities Management is responsible for installing signage in common areas (hallways, elevators, lobbies, etc.). You will need to post additional signage in your research spaces. Templates for locally printable research restart signage are now available for download.
- Animal Research
-
Prior to restarting animal research, review the following:
- DLAR guidelines for restarting animal research and scheduling facility. Contact the DLAR Director, Dr. Frank Jenkins with questions.
- How to secure an IACUC approval
- Human Subject Research
-
Research involving human subjects can restart. The Human Research Protection Office has created a survey to determine how the COVID-19 restrictions can be lifted from human research studies to restart.
To determine if your human research studies can restart, complete the SURVEY to DETERMINE HUMAN RESEARCH START-UP as defined in this guide.
The Human Research Protection Office provides information on how to secure an IRB approval.
For questions related to the survey or restarting human subject research, contact askirb@pitt.edu.
- Travel, Visitors, and Vendors
-
Updated travel guidance is forthcoming.
You may need to bring a service technician or field engineer to repair equipment in your lab. Vendors are required to complete a Provider Covenant to Comply with COVID-19 Policies and Procedures. If you have questions related to vendors on campus, contact Panther Express Customer Service.
COVID-19 Communications and News
- September 10, 2020: A Message from Provost Ann E. Cudd
- July 17, 2020: From the Chancellor: Resilience Framework July 17 Update
- July 1, 2020: From the Human Resources Office: Update to Staff: Plans for Being Safe on Campus
- June 30, 2020: From the Chancellor: Our plans for being safe on campus
- June 17, 2020: Pittwire: University Draws on Own Experts to Guide Health and Safety Decisions
- June 3, 2020: SVC Research: Research Restart Website